Abstract
Background: The myeloproliferative neoplasms (MPN) and myelodysplastic syndrome (MDS) are usually sporadic diseases, however, familial cases are well-described. Approximately 10% of MPN cases display familial clustering, and there is a 5-7 fold increased risk of developing an MPN among first degree relatives of MPN patients. While familial MDS appears to be less common, identification of multiple predisposition genes has led to the incorporation of this entity into the most recent WHO classification of myeloid neoplasms. Identification of predisposing germline mutations in the myeloid malignancies has led to a better understanding of the pathophysiology of these diseases and improved clinical care. However, many familial MDS and MPN cases exist in which the inherited genetic lesion is unknown. Our recent investigation of a multigenerational family with inherited MDS/AML marked by erythroid hyperplasia identified a missense variant in ERBB3 that co-segregated with the disease phenotype (1). In this study, we investigated a kindred with an autosomal dominant familial cancer syndrome characterized by both MPN and solid tumor malignancies.
Methods: Whole exome sequencing was performed via the Baylor-Hopkins Center for Mendelian Genomics at Center for Inherited Disease Research at Johns Hopkins University. Saliva or peripheral blood samples were used as source DNA. Sequence analysis and variant calling/filtering was performed as previously described (1). Targeted sequencing of the ERBB genes was performed via MiSeq using an Illumina TruSeq Custom Amplicon (v1.5) designed to sequence the entire open reading frame of selected genes. Analysis was performed using Illumina BaseSpace TruSeq applications. Germline variants were identified via variant allele frequency and heterozygous, nonsynonymous variants with a minor allele frequency < 0.01 and a depth > 40x were prioritized.
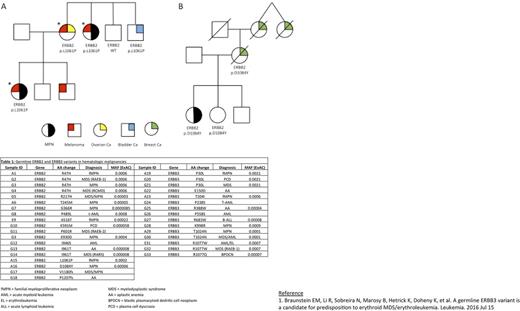
Results: The proband was diagnosed with a JAK2 V617F positive MPN at age 37. Subsequent personal cancer history included melanoma and renal carcinoma. Family history was significant for ovarian cancer and melanoma in her mother, MPN and melanoma in her maternal aunt, and bladder cancer in her maternal uncle (Figure 1A). Exome sequencing of the three family members affected with melanoma was performed in order to identify the predisposing inherited variant in this family. Sequence analysis produced 71 candidate rare, heterozygous, nonsynonymous single nucleotide variants (SNV), including one in the gene ERBB2 . Genotyping of 2 additional family members for the ERBB2 c.3182T>C;p.L1061P SNV established that it co-segregates with disease (Figure 1A). To further investigate the prevalence of rare germline ERBB2 and ERBB3 variants in both MPN and MDS, we performed targeted sequencing of these genes in two discovery cohorts: 1) 96 cases of MPN, of which 63 had a 1st or 2nd degree relative with a family history of MPN and 2) 19 cases of erythroid MDS/AML. Five out of 96 (5.2%) cases of MPN were found to have ERBB2 variants, while 3 of 96 (3.1%) had ERBB3 variants. Of the 19 erythroid MDS/AML cases, no ERBB2 variants were identified, while 1 had an ERBB3 variant. Further, one MPN case with a germline ERBB2 c.G3250T:p.D1084Y variant had a family history of breast cancer in her mother, who also carried this variant (Figure 1B). We analyzed a validation cohort of 630 cases of hematologic malignancies (approximately 20% MPN) sequenced as part of their clinical evaluation at the Sidney Kimmel Cancer Center. Similar to the findings in our discovery cohorts, rare germline ERBB2 variants were enriched in MPN, found in approximately 5% of MPN compared to only 1.8% of all other myeloid disorder diagnoses. In contrast, germline ERBB3 variants were present in 1.6% of MPN compared to 1.8% of the other myeloid disorders (Table).
Conclusions: We have identified germline ERBB2 variants that co-segregate with disease in two kindreds with familial cancer syndromes manifesting as a mixed MPN and solid tumor phenotype. In addition, we show that germline ERBB2 and ERBB3 variants are present in patients with hematologic malignancies at a higher incidence than expected in the general population, and ERBB2 variants are associated with an MPN phenotype. Together, these data implicate these genes in the predisposition to myeloid malignancy as well as other familial cancer syndromes.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal